Industries such as pharmaceuticals, biotechnology, and electronics operate under strict contamination control standards. Their success depends on cleanroom equipment environments that deliver not only precision but also compliance with ISO and GMP regulations.
In this article, we explore how AGMM TECH provides specialized cleanroom equipment to meet the specific demands of these high-stakes sectors—from sterility assurance to particle control—while ensuring flexibility and long-term operational efficiency.
Requirements for Pharma and Biotech Facilities
Pharmaceutical and biotech companies must operate under stringent Good Manufacturing Practices (GMP) and ISO 14644-1 Pharmaceutical and biotech companies must operate under stringent Good Manufacturing Practices (GMP) and ISO 14644-1 cleanroom classifications, where product integrity, patient safety, and regulatory compliance are non-negotiable. These industries rely heavily on contamination control systems that support aseptic workflows, minimize particulate levels, and ensure reproducibility across all production batches.
The cleanroom equipment used must enable precise environmental control throughout every stage of drug or biologic development, including formulation, filling, packaging, and quality control. Additionally, the equipment must be compatible with regulatory inspections, traceability requirements, and standard operating procedures (SOPs) that govern pharmaceutical-grade environments.
Key Requirements Include:
- ISO Class 5 or better in aseptic filling areas, where sterile conditions must be guaranteed at all times during the handling of open or exposed products.
- Positive pressure environments to prevent backflow of contaminated air, especially in Grade A and B zones, ensuring product protection from external sources.
- HEPA-filtered laminar airflow to provide a unidirectional stream of sterile air across critical work surfaces, maintaining consistent air quality and eliminating dead zones.
- Validated decontamination processes for air showers, isolators, pass boxes, and cleanroom hoods, all of which must undergo IQ/OQ protocols and be auditable.
- GMP-compliant documentation including technical files, calibration reports, CE marking, and cleaning validation protocols that support regulatory approval processes.
Furthermore, equipment in pharma and biotech environments must be constructed with non-porous, corrosion-resistant materials like 304 or 316L stainless steel, featuring smooth welds and seamless corners to prevent microbial accumulation and facilitate disinfection. Integration with BMS (Building Management Systems) or PLC-based controls is also essential for real-time environmental monitoring, alarm systems, and process validation.
AGMM TECH addresses all these requirements with precision-engineered equipment, designed to meet the critical demands of pharmaceutical and biotech production with full regulatory alignment and operational reliability.
H2: Cleanroom Challenges in Microelectronics
The microelectronics and semiconductor industries demand an ultra-clean manufacturing environment where even sub-micron particles can cause defects, reduce yield, or compromise device functionality. In contrast to pharmaceutical cleanrooms—where microbial control is the top concern—microelectronics cleanrooms focus on strict particulate management, static control, and environmental precision.
In this sector, the smallest airborne particle can settle on wafers during lithography or deposition processes and permanently impair electrical conductivity, rendering chips unusable. As a result, these facilities must enforce contamination control with surgical precision, especially in high-value operations like wafer fabrication, MEMS manufacturing, and optical sensor assembly.
Main Challenges Include:
- Maintaining ISO Class 4–6 environments throughout key production zones. These classifications allow no more than 10 to 1,000 particles ≥0.1 micron per cubic meter—requiring advanced filtration and airflow regulation.
- Managing static electricity and humidity, both of which can damage sensitive microchips and circuits. ESD-safe materials and humidity-controlled enclosures are essential to prevent electrostatic discharge and condensation-related failures.
- Preventing particle dispersion from equipment and operators, which account for a significant portion of in-process contamination. This involves selecting cleanroom-compatible machinery, installing shielding components, and enforcing strict gowning protocols.
- Ensuring zoned air control for specific processes like photolithography, etching, and assembly. Each process may demand different airflow velocity, pressure differentials, or temperature settings.
AGMM TECH addresses these technical and environmental challenges through a range of cleanroom solutions specifically engineered for the microelectronics sector:
- Ultra-clean Fan Filter Units (FFUs) with ULPA filtration for ISO Class 4 applications
- Custom pass boxes and trolleys designed to prevent vibration, particulate dispersion, and electrostatic buildup during material transfers
- Air showers with high-velocity airflow jets, ensuring that operators and tools entering clean zones are thoroughly decontaminated
In addition, all AGMM TECH cleanroom components can be configured with anti-static finishes, humidity monitoring systems, and smart PLC controls to deliver granular environmental management. This makes AGMM TECH a trusted partner in high-tech industries where quality depends on absolute cleanliness, precision, and environmental stability.
H2: Matching Equipment to Industry-Specific Needs
Cleanroom operations differ significantly across industries, and selecting the right equipment requires a deep understanding of each sector’s regulatory, technical, and process-driven requirements. AGMM TECH addresses these complexities by offering a full portfolio of modular, customizable cleanroom equipment designed to support both sterile production and precision assembly, while ensuring long-term compliance with GMP and ISO 14644-1 standards.
Our approach emphasizes equipment scalability, layout adaptability, and validation readiness, enabling seamless integration into both new builds and retrofit installations.
Pharmaceutical & Biotech Equipment:
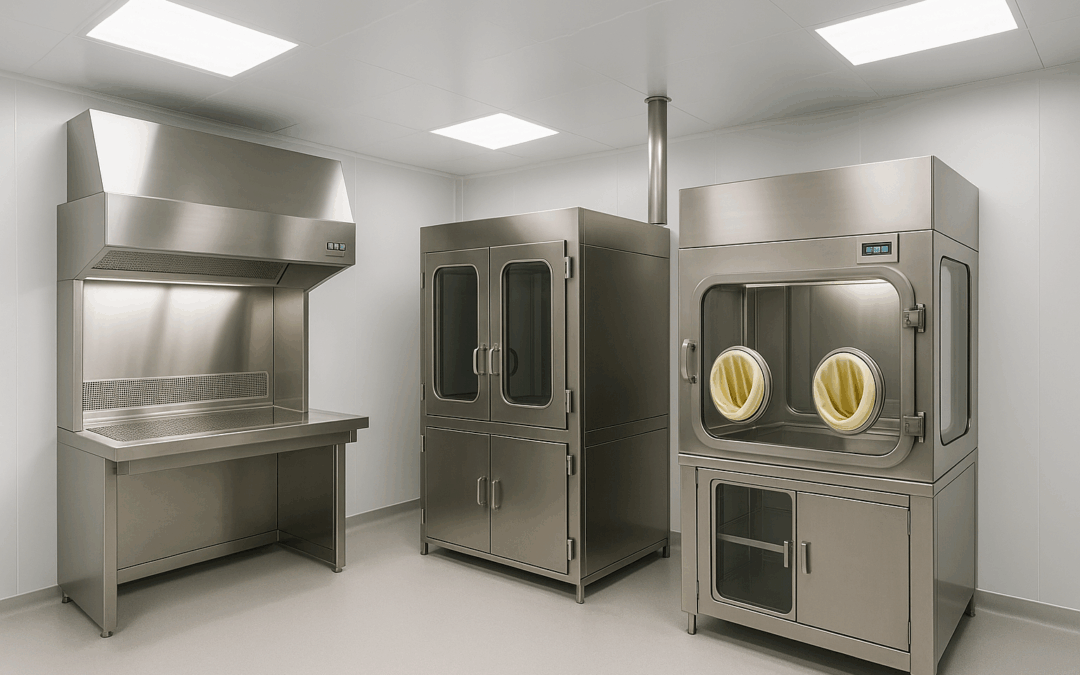
- Positive Pressure Isolators: Designed for aseptic drug manufacturing, these isolators protect the sterile product from environmental contamination through outward filtered airflow and maintain Grade A conditions inside the working chamber.
- Air Showers: Installed at entry points to Grade A/B zones, air showers remove particles from garments and carts, helping prevent cross-contamination during personnel or material transfer.
- Vertical Laminar Flow Hoods: Provide unidirectional HEPA-filtered airflow for sterile compounding, IV preparation, and microbiological quality control.
- Dynamic Pass Boxes: Enable secure material transfer between zones without personnel movement, featuring interlocking doors and internal HEPA filtration for ISO-compliant conditions.
Microelectronics Equipment:
- Fan Filter Units (FFUs) with ULPA Filters: Deliver ultra-clean airflow necessary for ISO Class 4 environments used in wafer fabrication, MEMS assembly, and photolithography.
- Horizontal Laminar Flow Hoods: Support optical alignment and sensor integration processes by maintaining clean airflow across wide work surfaces with minimal turbulence.
- Custom Cleanroom Furniture: Includes ESD-safe workbenches, shelving, and carts designed to reduce static discharge and particle accumulation during sensitive operations.
Medical Devices & Optics:
- Powder Hoods: Offer containment for dry powders and light particulate substances used in device formulation or coating processes.
- UV-Safe Environments: Shield light-sensitive components during assembly and testing, ensuring compliance with optical device production requirements.
- Flexible Modular Cleanrooms: Built to accommodate calibration benches, packaging lines, and Class 7–8 zones used in final device inspection or assembly.
- Pressure-Zoned Cleanrooms: Create logical separation between sterile zones and general assembly areas to prevent cross-contamination in mixed-use spaces.
Across all applications, AGMM TECH delivers:
- Comprehensive validation packages with IQ/OQ documentation
- Integration with Building Management Systems (BMS) or PLC control platforms
- Support for CE marking, ATEX compliance, and traceability documentation
Whether your focus is sterile injectable production, microchip fabrication, or surgical implant assembly, AGMM TECH provides the tailored cleanroom infrastructure needed to keep your operations compliant, efficient, and future-proof.
H2: Sector Applications of AGMM TECH Solutions
AGMM TECH cleanroom equipment is currently in use across several advanced manufacturing and healthcare sectors. Here are real-world applications where our systems make a measurable difference:
Pharma & Biotech:
- Sterile compounding in hospital pharmacies
- GMP production suites for injectable drugs and biologics
- Gene therapy labs requiring custom isolators with precise pressure differentials
Electronics & Optics:
- MEMS and sensor assembly lines using modular ISO Class 5 cleanrooms
- Wafer manufacturing supported by ceiling-mounted FFUs
- Optical lens assembly using horizontal laminar airflow units
Medical Devices:
- Surgical tool packaging lines inside softwall cleanrooms
- Implant production under controlled airflow and particulate zones
Food & Beverage:
- Sterile bottling lines protected with air showers and pass boxes
- Packaging areas isolated with modular GMP enclosures
Each AGMM TECH solution is tailored to the facility’s layout, ISO target, and production requirements, making our systems versatile across continents and compliance frameworks.
These applications highlight the flexibility and technical precision of AGMM TECH systems. From mobile labs in emerging markets to high-volume European production plants, our cleanroom equipment is engineered for global scalability.
Clients benefit from fast installation, seamless integration with automation systems, and long-term regulatory support. Regardless of sector, AGMM TECH delivers reliability where contamination control is mission-critical.
Conclusion
The pharmaceutical, biotech, and electronics sectors demand uncompromising cleanroom environments that combine sterility, precision, and flexibility. AGMM TECH delivers equipment that meets the highest ISO and GMP standards, offering scalable, modular solutions that align with your workflow and future expansion goals. Whether you’re launching a new production line or upgrading an existing facility, our expertise ensures that your cleanroom remains compliant, high-performing, and built to support your mission—today and tomorrow.